Regenerative Medicine
Exosome Therapy
Bio-Hacked Solutions for Age-Defying, Clinically Advanced Results
WHY EVO BIOLOGIX?
At Evo Biologix, we connect top-tier practitioners to the most advanced exosome therapies available—powered by the placental MSC secretome from a single, pre-pandemic master cell line. Designed for topical, subcutaneous, and intravenous delivery, our products are lab-engineered, highly concentrated, and free of variability—ensuring safety, potency, and repeatable outcomes.
No blood draws. No guesswork. Just cellular reprogramming at the molecular level.
Why Evo Biologix:
At Evo Biologix, we connect top-tier practitioners to the most advanced exosome therapies available—powered by the placental MSC secretome from a single, pre-pandemic master cell line. Designed for topical, subcutaneous, and intravenous delivery, our products are lab-engineered, highly concentrated, and free of variability—ensuring safety, potency, and repeatable outcomes.
No blood draws. No guesswork. Just cellular reprogramming at the molecular level.
THE SCIENCE: Small Vesicles. Big Change.
What Are Exosomes? Nano-sized extracellular vesicles (30–150 nm) released by mesenchymal stem cells (MSCs), exosomes act as biological messengers—delivering RNA, mRNA, growth factors, proteins, peptides, and lipids to surrounding cells. These “instructions” promote: https://www.kimeralabs.com/exosomes/#
LEARN MORE:
-
By down-regulating inflammatory proteins like TNF-a, IL-1β and MMPs, and upgregulating anti-inflammatory proteins like IL-4, IL-10 and TIMPs, MSC exosomes could reduce inflammation, which is central mechanism of many autoimmune, inflammatory and degenerative conditions.
-
By supplying diseased or degenerative cells with proteins and RNA that support essential cellular functions, MSC exosomes could exogenously maintain cell viability and prevent apoptosis. The anti-apoptotic message contained within these exosomes may help to slow, stop or reverse tissue degeneration.
-
Exosomal proteins such as peroxiredoxins could reduce the oxidation state of cells under oxidative stress, which is present in many disease states and plays a role in tissue degeneration associated with aging. Reducing the oxidative stress on cells could improve their function and survival.
-
In preclinical studies, MCS exosomes optimize tissue remodeling in damaged skin and other tissues with improved type I/III collagen ratio and normal organization of collagen fibers. Reducing fibrosis during wound healing or after tissue injury could improve scar formation and tissues.
-
By polarizing immune cells, such as macrophages and T-cells, to less inflammatory and more regulatory phenotypes, MSC exposes could modulate the activity of immune cells to improve autoimmune disease and mitigate conditions characterized by excessive immune response.
-
By stimulating endothelial cell function, MCS exosomes could improve angiogenesis. Improving the blood supply to injured or diseased tissues could help to increase the survival of damaged cells and prevent tissue degeneration and impaired function.
-
Activation of specific cells, such as fibroblasts, by MSC exosmes could enhance extracellular matrix production and help to restore damaged tissues. Specific exosome microRNA molecules could also help to regenerate normal collagen in the skin or stimulate production of normal articular cartilage.
-
Proteins like histone deacetylases contained within MSC exosomes may induce transcription of inactive DNA and influence the functions of target cells without altering the genome. By altering the methylation patterns of DNA, dormant gene expression could be re-activated.
Our exosomes are proven to cross the blood-brain barrier (BBB), opening up new possibilities for IV and systemic wellness therapies.
Unlike PRP or PDGF—which are limited in complexity and depend on patient factors—our placenta-derived exosomes offer a multifactorial regenerative signal, validated by third-party testing and verified for CD9, CD63, and CD81 markers.
BEYOND PRP & PDGF
CLINICAL APPLICATIONS
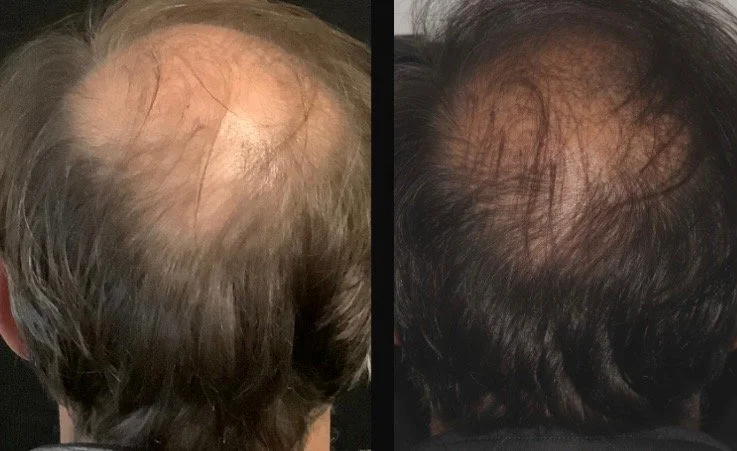
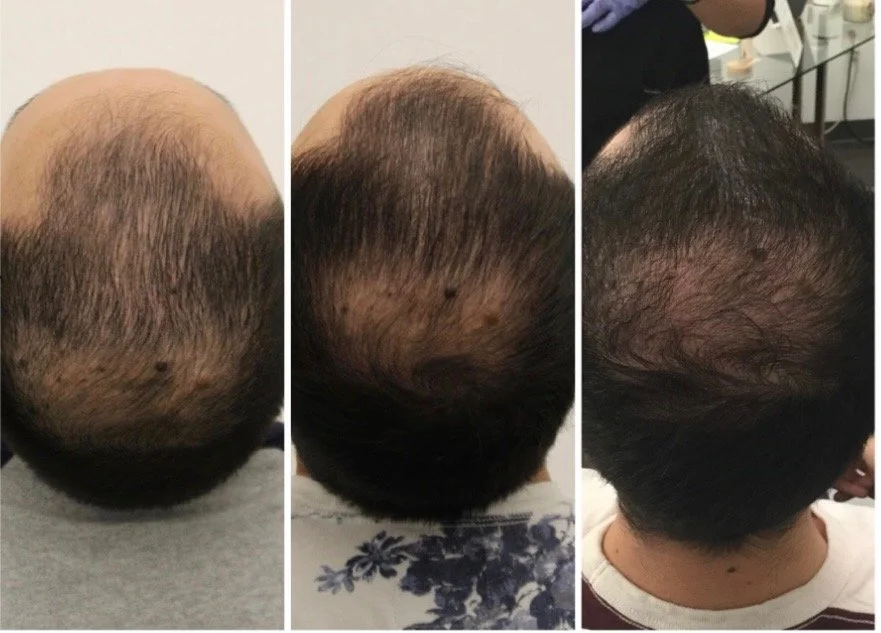
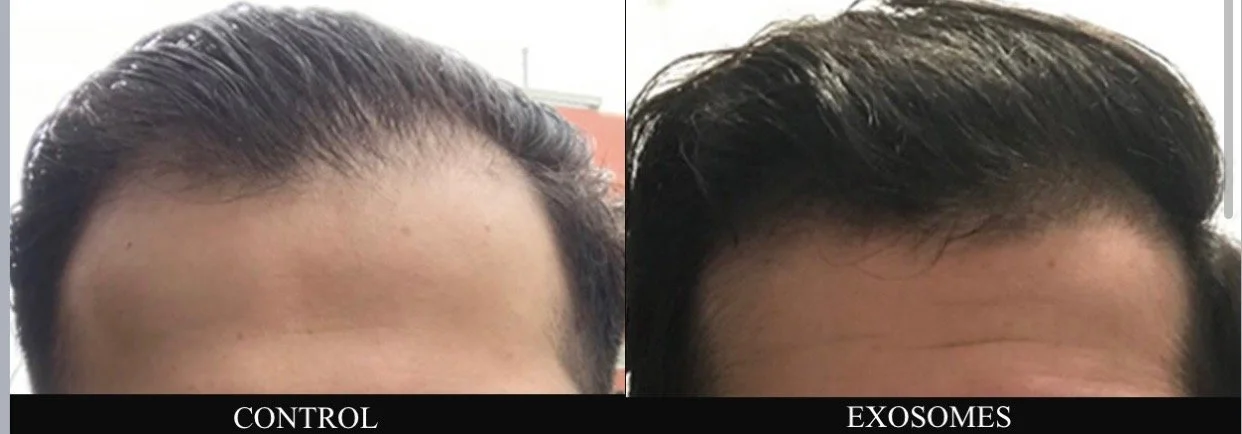
Hair Restoration (Male/Female Pattern, Telogen Effluvium)
Post-Procedure Recovery (Laser, Microneedling, Microdermabrasion)
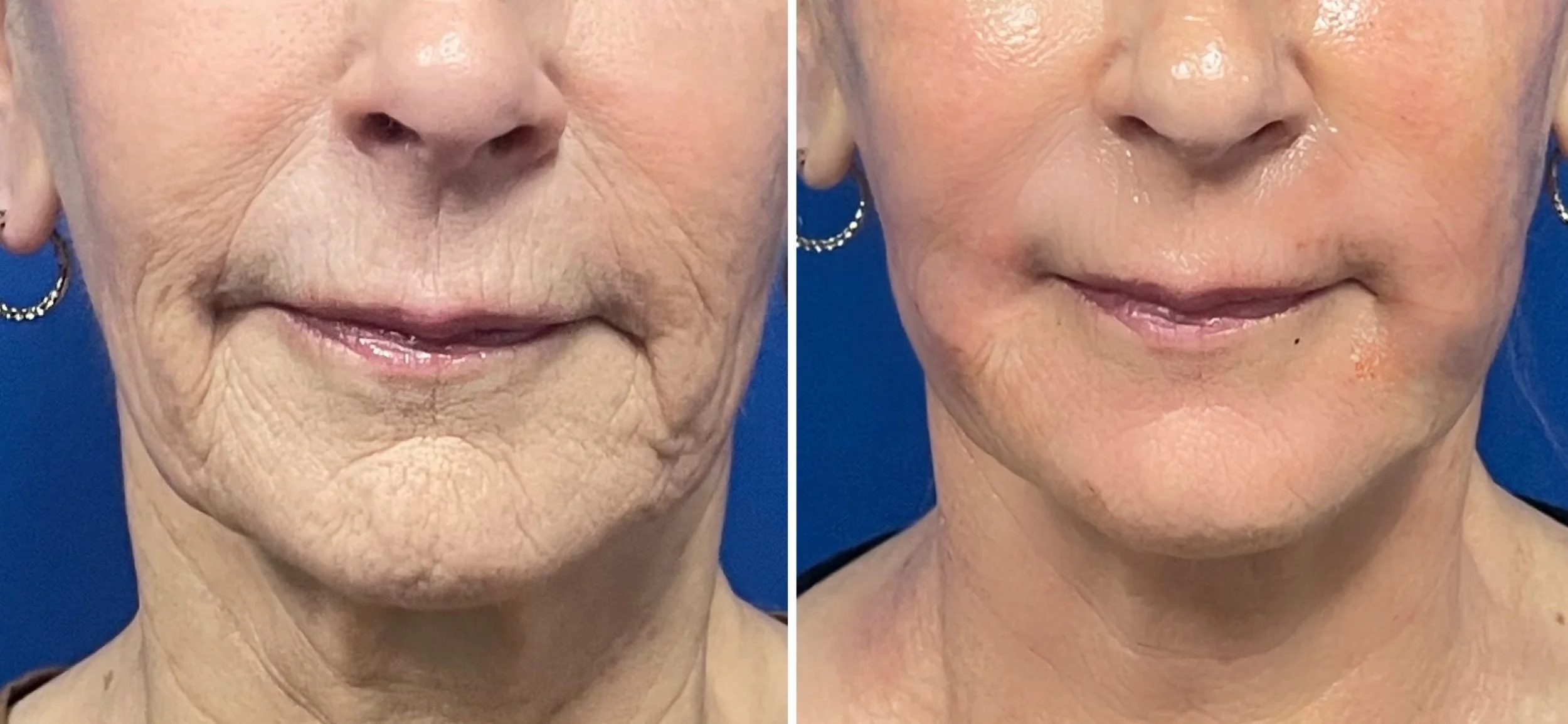
Scarring & Skin Health (Stretch marks, acne scars, surgical sites)
OUR EXOSOME PRODUCT LINE
INTRAVENOUS
5 to 15 trillion microvesicles per vial
Engineered for maximum purity, potency, and immune tolerance
Designed for intravenous and subcutaneous use
SUBCUTANEOUS
EV-rich solution for hair and scalp regeneration
Activates Wnt/β-catenin signaling for follicle reactivation
Stimulates circulation and dermal papilla cells
TOPICAL
Topical exosome-based formula with hyaluronic acid, trehalose, and amino acids
Calms and hydrates skin while enhancing regenerative response post-procedure
WHY WE’RE DIFFERENT
Single Donor, Pre-COVID Master Cell Line: Ensures purity, consistency, and absence of viral proteins
FDA-Inspected Lab: Manufactured in a 27,000 sq. ft. cleanroom-certified facility
Independent Verification: Over 60x RNA signal compared to competitors
DSTORM Microscopy: Confirms presence of actual exosomes vs. cell debris
CD9/CD63/CD81 Positive: Biomarker-confirmed purity
-
NTA and DLS are both techniques utilized in the Research and Development (R&D) and Quality Control (QC) laboratories for the analysis and characterization of nanoparticles in solution. NTA analysis is performed using a Malvern Panalytical NanoSight NS300 instrument, while the DLS analysis is performed using the DynaPro® NanoStar® instrument. Both instruments accurately measure the size distribution and concentration of all types of nanoparticles; however, the DynaPro® NanoStar® can differentiate between particle families, and can also be used for proteins, quantum dots, liposomes, metallic nanoparticles, and other types of particle analysis.
-
This state-of-the-art super-resolution microscope can capture images of a single extracellular vesicle (EV) in solution. Both, MSCs used in the manufacturing process as well as exosomes manufactured at Kimera Labs are characterized by different means, and nothing captures better multi-color 3D images at 100x resolution than our ONI super-resolution microscope.
-
The Pico is a cell imaging system used at Kimera Labs for cell characterization, as well as for potency assay testing analysis. Whether running fluorescence imaging or brightfield assays, the automated imager has preconfigured protocols that can be used for the development of cell-based assays, which have proven extremely helpful for R&D studies.
-
HPLC is a technique used for the characterization of the EVs in solution, as well as to determine the concentration and purity of the product. Precise and accurate characterization of the finished vialed product is performed by Size Exclusion Chromatography (SEC) using our Agilent® 1260 Infinity II HPLCs which are equipped with both Diode Array Detector as well as Fluorescence Detector.
-
Western Blot technique is used to determine the presence of surface markers commonly found on EVs. As part of the characterization and analysis of each EV-lots manufactured at Kimera Labs, identification of these markers (e.g. CD81, DC63) by WB is a routine technique utilized to support the identification and concentration of the product.
-
One of the most commonly used techniques for the isolation, purification, and characterization of different biological products is differential centrifugation. Ultracentrifugation is widely used in the purification and characterization of EVs. In our R&D lab, ultracentrifugation techniques have enabled us to continue improving our processes and methods used for EV analysis and characterization.
-
RT-PCR is one of the most sensitive techniques for the detection, quantitation, and characterization of RNA currently used in laboratories worldwide. RT-PCR is used at Kimera Labs to accurately characterize the cargo of our EVs. RT-PCR is used to support the analysis of our potency assays, as well as to effectively and accurately compare the RNA cargo of each lot manufactured at our facility.
-
Our Illumina NextSeq 1000 System, one of the newest and most advanced sequencing instruments available in the market enables us to accurately determine the exact RNA cargo and concentration in our EV products. Our NextSeq 1000 System allows us to accurately use this technology in the analysis and characterization of EVs, while enabling the development of products that perhaps a few years ago were not even imaginable. When samples are processed in our NextSeq 1000 System, we can accurately determine the exact sequence of each of the cargo RNA, as well as the concentration of each specific RNA family. This further assures that our processes are under control, and the quality of the product is been maintained from lot to lot.
-
At Kimera Labs, the entire cGMP manufacturing process (upstream and downstream) is conducted inside our ISO 7 certified cleanrooms, which contain multiple ISO 5 certified Laminar Flow Glovebox Isolators (LFGIs). ISO certification not only ensures compliance with the most rigorous environmental control standards but also helps to reduce the possibility of contaminating the product while been processed. From procedure-driven routine and meticulous aseptic cleaning of the rooms to recurring environmental monitoring, nothing is left to chance when the sterility and quality of the product are at stake. The room as well as the LFGIs are equipped with High Efficiency Particulate Air (HEPA) filter, which traps particles that are 0.3 microns and larger. Multiple UV-C lights in the room, inside the LFGIs, as well than in the AC ducts are used to further assist in maintaining the sterility of the room.
READY TO BRING THIS TO YOUR PATIENTS?
We’d love to schedule a 30-minute call with our Chief Science Officer to explore how exosome therapy can elevate your current treatment offerings, from aesthetics to systemic regeneration.
Clinical Questions? We’re Here to Support You.
Submit your inquiry and one of our clinical support specialists will be in touch shortly.
DISCLAIMERS
For U.S. practitioners only. Products are intended for cosmetic topical use, site-specific subcutaneous injection, or intravenous delivery. These are not drug products and are not intended to diagnose, treat, or cure any disease. All materials are derived from a single pre-COVID placental MSC source and produced under FDA-inspected conditions.